당신은 주제를 찾고 있습니까 “ge b40 monitor service manual – GE | Monitor B40 and B40i“? 다음 카테고리의 웹사이트 https://ppa.charoenmotorcycles.com 에서 귀하의 모든 질문에 답변해 드립니다: https://ppa.charoenmotorcycles.com/blog/. 바로 아래에서 답을 찾을 수 있습니다. 작성자 idsMED 이(가) 작성한 기사에는 조회수 4,535회 및 좋아요 13개 개의 좋아요가 있습니다.
ge b40 monitor service manual 주제에 대한 동영상 보기
여기에서 이 주제에 대한 비디오를 시청하십시오. 주의 깊게 살펴보고 읽고 있는 내용에 대한 피드백을 제공하세요!
d여기에서 GE | Monitor B40 and B40i – ge b40 monitor service manual 주제에 대한 세부정보를 참조하세요
ge b40 monitor service manual 주제에 대한 자세한 내용은 여기를 참조하세요.
B40/B20 Patient Monitor User’s Guide – Tiger Medical
In this manual, the B40 Patient Monitor and B20 Patient Monitor are … are required, contact GE service for technical support or contact.
Source: image.tigermedical.com
Date Published: 12/15/2021
View: 8407
PROCARE* Monitor B40/B20 Technical Reference Manual
GE Healthcare. PROCARE* Monitor B40/B20. Technical Reference Manual. PROCARE Monitor B40/B20. English. 2050802-001 C (Paper).
Source: diamedicalusa.com
Date Published: 2/29/2022
View: 2773
GEHC Service Manual PROCARE B40 B20 Monitor
8/11/2019 GEHC Service Manual PROCARE B40 B20 Monitor 1/252GE HealthcarePROCARE* … GE Healthcare3F Building 1, GE Technology Park1 Huatuo RoadShanghai PRC …
Source: fdocuments.in
Date Published: 12/26/2022
View: 6741
GE B40 Technical Reference Manual – PDFCOFFEE.COM
Service Menu iii Document no. 2062472-001. B40 Patient Monitor 1. Introduction 1.1 1.2 2 3. SW Management 3 2.1 2.2 2.3 2.4 2.5 2.6 3 4 4 5 5 5
Source: pdfcoffee.com
Date Published: 5/2/2021
View: 237
GEHC Service Manual – PROCARE B40 B20 Monitor | PDF
GEHC-Service-Manual_PROCARE-B40-B20-Monitor.pdf – Free ebook download as PDF … GE Healthcare3F Building 1, GE Technology Park1 Huatuo RoadShanghai PRC …
Source: www.scribd.com
Date Published: 6/6/2021
View: 3675
Ge | Monitor B40 And B40I 54 개의 새로운 답변이 업데이트 …
PAGE 5 About this manual Monitor naming … contact GE service for technical support or …
Source: ppa.covadoc.vn
Date Published: 5/28/2022
View: 8097
PROCARE* Monitor B40/B20 Technical Reference
GE Healthcare PROCARE* Monitor B40/B20 Technical Reference Manual PROCARE Monitor B40/B20 English 2050802-001 C (Paper) © 2011 General Electric Company.
Source: dokumen.tips
Date Published: 3/16/2021
View: 6322
PROCARE* Monitor B40/B20 User’s Reference Manual
GE Medical Systems Information Technologies, Inc. … The B40/B20 Patient Monior monitors and displays oscillometric non-invasive blood …
Source: discountcardiology.com
Date Published: 8/15/2022
View: 5582
주제와 관련된 이미지 ge b40 monitor service manual
주제와 관련된 더 많은 사진을 참조하십시오 GE | Monitor B40 and B40i. 댓글에서 더 많은 관련 이미지를 보거나 필요한 경우 더 많은 관련 기사를 볼 수 있습니다.
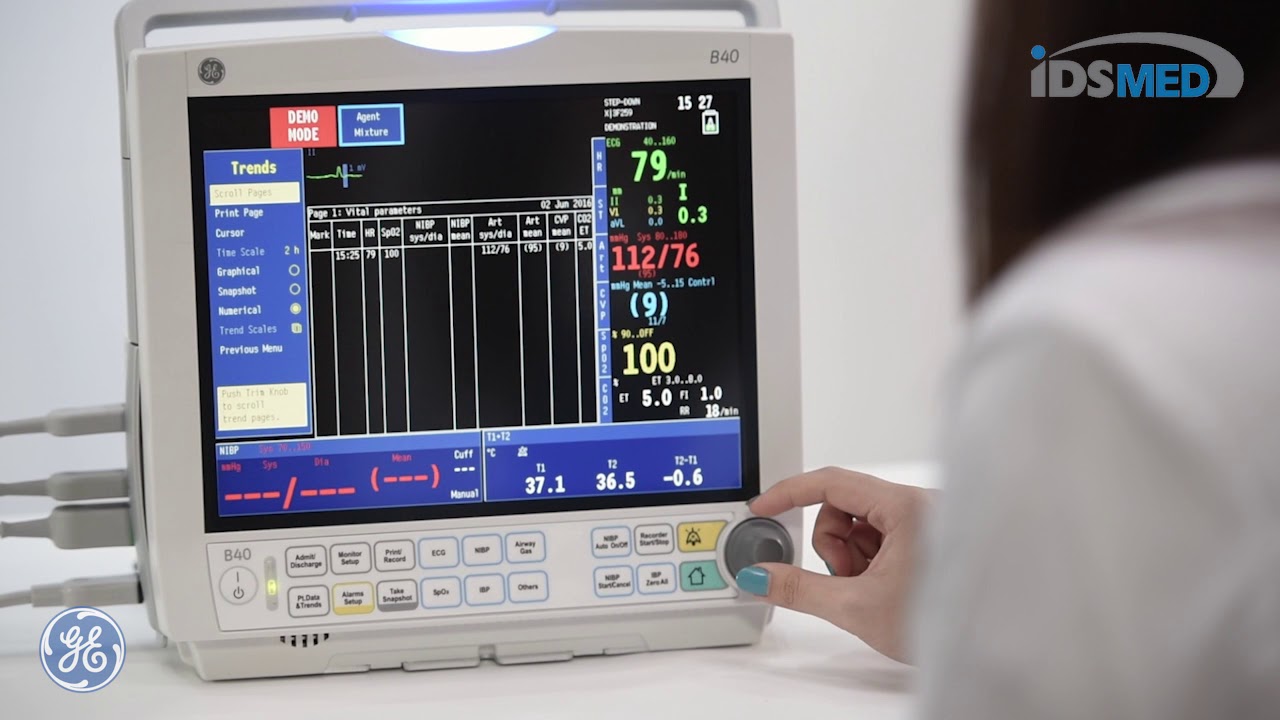
주제에 대한 기사 평가 ge b40 monitor service manual
- Author: idsMED
- Views: 조회수 4,535회
- Likes: 좋아요 13개
- Date Published: 2017. 11. 15.
- Video Url link: https://www.youtube.com/watch?v=oErp2TlZVR8
GEHC Service Manual PROCARE B40 B20 Monitor
All specifications are subject to change without notice.
Conformity according to the Council Directive 93/42/EEC concerning Medical Devices.
8/11/2019 GEHC Service Manual PROCARE B40 B20 Monitor
4/252
ClassificationsIn accordance with IEC 60601-1
Class I and internally powered equipment – the type of protection against electric shock.Type BF or CF equipment. The degree of protection against electric shock is indicated bya symbol on each parameter module.Equipment is not suitable for use in the presence of a flammable anesthetic mixture withair or with oxygen or nitrous oxide.Continuous operation according to the mode of operation.Portable Monitor
In accordance with IEC 60529IP21 – degree of protection against harmful ingress of water.
In accordance with EU Medical Device DirectiveIIb.
In accordance with CISPR 11:Group 1 Class A;
Group 1 contains all ISM (Industrial, scientific and medical) equipment in which thereis intentionally generated and/or used conductively coupled radio-frequencyenergy which is necessary for the internal functioning of the equipment itself.
Class A equipment is equipment suitable for use in all establishments other thandomestic and those directly connected to a low-voltage power supply networkwhich supplies buildings used for domestic purposes.
Trademarks
Dash, PROCARE, DINAMAP, EK-Pro, Trim Knob, Unity Network, Datex, Ohmeda, S/5, D-fend, D-fend+, Mini D-fend, OxyTip+, EarSat, FingerSat, FlexSat are trademarks of GE Healthcare. Allother product and company names are property of their respective owners.
GEHC Service Manual – PROCARE B40 B20 Monitor
0% 0% found this document useful, Mark this document as useful
100% 100% found this document not useful, Mark this document as not useful
Ge B40 Monitor Service Manual | Ge | Monitor B40 And B40I 54 개의 새로운 답변이 업데이트되었습니다.
당신은 주제를 찾고 있습니까 “ge b40 monitor service manual – GE | Monitor B40 and B40i“? 다음 카테고리의 웹사이트 ppa.covadoc.vn 에서 귀하의 모든 질문에 답변해 드립니다: https://ppa.covadoc.vn/blog. 바로 아래에서 답을 찾을 수 있습니다. 작성자 idsMED 이(가) 작성한 기사에는 조회수 4,507회 및 좋아요 13개 개의 좋아요가 있습니다.
여기에서 이 주제에 대한 비디오를 시청하십시오. 주의 깊게 살펴보고 읽고 있는 내용에 대한 피드백을 제공하세요!
In this manual, the B40 Patient Monitor and B20 Patient Monitor are … are required, contact GE service for technical support or contact.
+ 더 읽기
Source: image.tigermedical.com
Date Published: 12/29/2021
View: 4310
GE Healthcare. PROCARE* Monitor B40/B20. Technical Reference Manual. PROCARE Monitor B40/B20. English. 2050802-001 C (Paper).
+ 자세한 내용은 여기를 클릭하십시오
Source: diamedicalusa.com
Date Published: 12/6/2022
View: 1848
8/11/2019 GEHC Service Manual PROCARE B40 B20 Monitor 1/252GE HealthcarePROCARE* … GE Medical Systems Information Technologies, Inc.8200 West Tower …
+ 여기에 자세히 보기
Source: fdocuments.in
Date Published: 2/1/2021
View: 5304
Service Menu iii Document no. 2062472-001. B40 Patient Monitor 1. Introduction 1.1 1.2 2 3. SW Management 3 2.1 2.2 2.3 2.4 2.5 2.6 3 4 4 5 5 5
+ 여기에 더 보기
Source: pdfcoffee.com
Date Published: 11/11/2021
View: 6536
Copyright 2011 General Electric Company. All rights reserved. GE Healthcare 3F Building 1, GE Technology Park 1 Huatuo Road Shanghai PRC 201203. Tel: +86 21 …
+ 여기에 자세히 보기
Source: www.scribd.com
Date Published: 10/16/2021
View: 7161
GE Healthcare PROCARE* Monitor B40/B20 Technical Reference Manual PROCARE Monitor B40/B20 English 2050802-001 C (Paper) © 2011 General Electric Company.
+ 여기에 보기
Source: dokumen.tips
Date Published: 1/9/2022
View: 4057
4 Service If the product malfunctions or if assistance, service, or spare parts are required, contact GE service for technical support or contact your local …
+ 여기를 클릭
Source: www.manualshelf.com
Date Published: 9/1/2022
View: 2048
[Full Ebook PDF] Ge B40 Monitor Service Manual. DOWNLOAD >>> http://urlme.top/TRNuG · Image with caption: · https://wakelet.com/wake/xFoGoyXHKXYR_7PE8GBbX …+ 여기에 보기
Source: wakelet.com
Date Published: 4/25/2022
View: 3599
주제와 관련된 더 많은 사진을 참조하십시오 GE | Monitor B40 and B40i. 댓글에서 더 많은 관련 이미지를 보거나 필요한 경우 더 많은 관련 기사를 볼 수 있습니다.
Class A equipment is equipment suitable for use in all establishments other thandomestic and those directly connected to a low-voltage power supply networkwhich supplies buildings used for domestic purposes.
Group 1 contains all ISM (Industrial, scientific and medical) equipment in which thereis intentionally generated and/or used conductively coupled radio-frequencyenergy which is necessary for the internal functioning of the equipment itself.
Class I and internally powered equipment – the type of protection against electric shock.Type BF or CF equipment. The degree of protection against electric shock is indicated bya symbol on each parameter module.Equipment is not suitable for use in the presence of a flammable anesthetic mixture withair or with oxygen or nitrous oxide.Continuous operation according to the mode of operation.Portable Monitor
100% 100% found this document not useful, Mark this document as not useful
TrademarksDash, ProCare, DINAMAP, EK-Pro, Trim Knob, Unity Network, Datex, Ohmeda, S/5, D-fend, D-fend+, Mini D-fend, OxyTip+, EarSat, FingerSat, FlexSat are trademarks of GE Healthcare. All other product and company names are property of their respective owners.
Class A equipment is equipment suitable for use in all establishments other than domestic and those directly connected to a low-voltage power supply network which supplies buildings used for domestic purposes.
Group 1 contains all ISM (Industrial, scientific and medical) equipment in which there is intentionally generated and/or used conductively coupled radio-frequency energy which is necessary for the internal functioning of the equipment itself.
air or with oxygen or nitrous oxide. Continuous operation according to the mode of operation. Portable Monitor
a symbol on each parameter module. Equipment is not suitable for use in the presence of a flammable anesthetic mixture with
ClassificationsIn accordance with IEC 60601-1 Class I and internally powered equipment – the type of protection against electric shock. Type BF or CF equipment. The degree of protection against electric shock is indicated by
PAGE 1 B40/B20 Patient Monitor User’s Guide English 2081499-002 E (Paper) 21 November 2014 © 2014 General Electric Company. All rights reserved.
PAGE 3 B40/B20 Patient Monitor User’s Guide Related to software VSP-C 0459 All specifications are subject to change without notice. Document no. 2081499-002 E 21 November 2014 GE Medical Systems Information Technologies, Inc. 8200 West Tower Avenue Milwaukee, WI USA Zip: 53223 Tel: +1 414 355 5000 (outside US) 800 558 5102 (US only) Fax: +1 414 355 3790 www.gehealthcare.com Copyright © 2014 General Electric Company. All rights reserved.
PAGE 4 Contents About this manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 Impedance respiration . . . . . . . . . . . . . . . . . . . . . . . .79 About this device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 Pulse oximetry (SpO2) . . . . . . . . . . . . . . . . . . . . . . . . . .83 Safety precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 Non-invasive blood pressure (NIBP) . . . . . . . . . . . .89 System introduction . . . . . . . . . . . . . . .
PAGE 5 About this manual Monitor naming conventions This manual contains instructions necessary to operate this device in accordance with its functions and intended use. Descriptions refer to the software VSP-C In this manual, the B40 Patient Monitor and B20 Patient Monitor are referred to as “the monitor” when a function or a feature applies to both. For describing monitor-specific issues, the monitors are referred to as B40 and B20 respectively.
PAGE 6 2 Related documents All publications conform with the product specifications and applicable IEC publications on safety and essential performance of electromedical equipment as well as with applicable UL and CSA requirements valid at the time of printing.
PAGE 7 About this device Indications for use: B40 Indications for use: B20 This device is a portable multi-parameter unit to be used for monitoring and recording of, and to generate alarms for, multiple physiological parameters of adult, pediatric, and neonatal patients in a hospital environment and during intra-hospital transport.
PAGE 8 4 Service If the product malfunctions or if assistance, service, or spare parts are required, contact GE service for technical support or contact your local representative. It is helpful for you to duplicate the problem, check and confirm the operation of all accessories to ensure that they are not the cause of the problem. Responsibility of the manufacturer GE Medical Systems Information Technologies, Inc.
PAGE 9 Classifications CE marking information In accordance with IEC 60601-1: − Class I and internally powered equipment – the type of protection CE compliance The monitor bears CE Mark CE-0459 indicating its conformity with the provisions of the Council Directive 93/42/EEC concerning medical devices amended by 2007/47/EC and fulfills the essential requirements of Annex I of this directive. The country of manufacture can be found on the equipment labeling. against electric shock. − Type BF or CF equipment.
PAGE 10 6 Product Compliance • • • • • • • • • • • • • • • • • This equipment is suitable for connection to public mains as defined in CISPR 11. This Monitor conforms to general safety standard for medical devices to IEC 60601-1. This Monitor conforms to EMC safety standard to IEC 60601-1-2. This Monitor conforms to usability safety standard for medical devices to IEC 60601-1-6. The application of usability engineering to medical device conforms to IEC 62366.
PAGE 11 Safety precautions These safety messages refer to the entire system. The message specific to parts of the system can be found in the relevant section. Safety message signal words Safety message signal words designate the severity of a potential hazard. Danger Indicates a hazardous situation that, if not avoided, will result in death or serious injury. Warning Indicates a hazardous situation that, if not avoided, could result in death or serious injury.
PAGE 12 8 • • • • • • • • • • contact with line voltage by inserting metal objects, such as the pins of leadwires, into the sockets of the power cord by mistake. If liquid has accidentally entered the system or its parts, disconnect the power cord from the power supply and have the equipment serviced by authorized service personnel. If a service message appears, discontinue use as soon as possible and have the device repaired.
PAGE 13 System introduction Safety precautions System components Warnings NOTE: Your system may not include all these components. Consult your local representative for the available components. • • • • • All system devices must be connected to the same power supply circuit EXCESSIVE LEAKAGE CURRENT – Do not use a multiple socket outlet or extension cord.
PAGE 14 10 Frame front view 2 1 11 10 9 5 8 4 7 B40 Front view 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11.
PAGE 15 Frame back view 1 6 4 3 5 7 2 Input/Output adapter and connectors 1. 2. 3. 4. 5. 6. 7. B40 back view Receptacle for power cord Serial port Defibrillator connector Nurse call connector Network connector Equipotential connector Multi I/O connector NOTE: 2,3,4 is on the multiple Input/Output adapter.
PAGE 16 12 Acquisition modules E-miniC module B40 acquisition modules: • • • • E-miniC E-sCO, E-sCAiO N-CAiO E-Entropy 2 1 B20 acquisition modules: • • E-miniC E-Entropy 3 1. Water trap 2. Sample gas inlet 3.
PAGE 17 E-sCO, E-sCAiO module N-CAiO module 1 2 2 3 1 3 4 4 E-sCAiO 1. 2. 3. 4. E-sCO 1. 2. 3. 4.
PAGE 18 14 E-Entropy module 1 2 1. Module keys 2.
PAGE 19 How to identify the Hemo connectors’ configuration The monitor provides different configurations for Hemo measurement. The user can identify Hemo connectors’ configuration from connectors and label.
PAGE 20 16 Command board keys 1 2a 3 4 5 Admit/ Discharge Monitor Setup Print/ Record ECG Take Snapshot SpO2 Pt.
PAGE 21 Symbols Type BF (IEC 60601-1) protection against electric shock. Isolated (floating) applied part suitable for intentional external and internal application to the patient, excluding direct cardiac application. − On the rear panel: − Electric shock hazard. Do not open the front or back cover. Servicing of the product should be performed only by qualified service personnel. − For continued protection against fire hazard, replace the fuse only with one of the same type and rating.
PAGE 22 18 On the screen: Audio alarms paused indicator – Indicates all audio alarms are paused and the amount of time remaining for the alarm pause period displays as a countdown timer. Displays in the upper left corner of the screen. In the front panel: battery. Equipotentiality. Monitor can be connected to potential equalization conductor. Alternating current. On the keypad: Bell cancel. Audio pause. Home. Return to the normal screen view. ON/OFF. Fuse. Gas inlet. Gas outlet.
PAGE 23 C 3ZG9 Underwriters Laboratories product certification mark. Medical Equipment with respect to electrical US shock, fire and mechanical hazards only in accordance with ANSI/AAMI ES60601-1:2005/ (R)2012 and A1:2012, C1:2009/(R)2012 and A2:2010/ (R)2012; CSA CAN/CSA-C22.2 NO. 60601-1:14; IEC 60601-2-26; IEC 60601-2-27; IEC 80601-2-30; IEC 60601-2-34; IEC 60601-2-49; ISO 80601-2-55; ISO80601-2-56; ISO80601-2-61 Rx Only U.S. Atmospheric pressure limitations. Recycled materials or may be recycled.
PAGE 24 20 Respiration indicator. Indicates a breath is detected by the impedence respiration algorithm. This symbol indicates that the waste of electrical and electronic equipment must not be disposed as unsorted municipal waste and must be collected separately. Please, contact an authorized representative of the manufacturer for information concerning the decommissioning of your equipment.
PAGE 25 Monitoring basics Warnings • • • • • • • Inserting and removing acquisition modules Ensure modules are oriented with release latch downward to ensure retention. The parameter modules are not able to withstand unpacked drops from a height of 1 m without damaging the module latches. If the device is dropped, please service the device before taking it back into use. After transferring or reinstalling equipment, always check that equipment is properly connected and parts are securely attached.
PAGE 26 22 Main screen layout Using menus The main screen displays alarms, trends, waveforms, digits, and the main menu in pre-defined areas. A menu is a list of functions or commands. To display a menu, press one of the keypad keys. To select menu options with Trim Knob control: 1. Rotate the Trim Knob control in either direction to move the highlighted cursor from option to option on the display. 2. Press the Trim Knob control once to select the highlighted option.
PAGE 27 Network Warnings On the S/5 network, the monitor is able to: • • • • • Do not use with iCentral software V5.0.3 and earlier. Do not use Mobile Care Server software V5.2 and earlier. Caution • Install HL7 network interfaces as specified, and only by qualified personnel. The monitor does not support the Patient Data Server (PDS). The monitor’s realtime patient data can’t be viewed on other monitors (e.g. Dash 3000/4000/5000, Solar 8000, B850, B650).
PAGE 28 24 Additionally, on the CARESCAPE network, the monitor supports remote parameter and waveform configurations from the central station. NOTES: • The Entropy parameter is not supported by CIC pro and CARESCAPE Central station. • On the iCentral, the care area settings can be setup to “Non OR” or “OR” in the DRI service menu. Please consult the authorized service personnel. Non OR: − Displays up to 72 hours of trend data.
PAGE 29 Batteries Warnings • • • • The monitor supports up to two lithium-ion batteries. Each battery can be charged separately. During charging, screen symbols and monitor frame LEDs indicate the current charging level and any possible failures. You can check the battery status through Monitor Setup – Battery Setup. For additional information please refer to the table following this statement. EXPLOSION OR FIRE-Using non-recommended batteries could result in injury/burns to the patients or users.
PAGE 30 26 Battery indicators Screen A B A Explanation B B B Front panel battery LEDs Checking the battery charge when the monitor is turned off Orange dark Monitor is battery Green lit powered. Batteries are fully charged; the size of the green bar indicates the charging level. Monitor is battery powered. Battery A is empty, battery B charge is partially charged. Orange dark Green lit Monitor is battery powered. Battery A failure, battery B is full.
PAGE 31 Replacing the batteries Battery capacity indicators in the upper right corner of the screen indicate when a battery needs to be replaced, charged, missing or not functional, see above table to additional information. CAUTION: After replacing a battery, always make sure to close the battery compartment by sliding the lid back to the right until it clicks. (1) Open the cover of the battery compartment located (2) Insert the new battery. Ensure that the battery on the side of the monitor.
PAGE 32 28 Troubleshooting • Battery operation time is markedly shortened: − Condition the batteries, see “Conditioning the batteries” and the “User’s Reference Manual.” • The monitor does not start: − Check that the batteries are properly inserted and sufficiently charged, see page 25. − Check that the power cord is properly connected. − Check the fuses and replace them if necessary, see “ Cleaning and care.
PAGE 33 Setting up the monitor before use Warnings • • Modifying the screen setup Operator should check the preset in use before use on each patient. A hazard can exist when using differing presets on like devices in a common area. For instance, two beside monitors used in an ICU with different presets may present a hazard. A graphic presentation of a generic layout can be found in the “Monitoring basics” chapter. When monitoring starts, the main page appears automatically.
PAGE 34 30 NOTE: Depending on your configuration, if the same measurement in the waveform field that is currently in the digit field, the digit field disappears. Changing split screen Selecting Combine Pressures in the Waveform Fields menu displays invasive pressures in the same waveform field with individual scales.
PAGE 35 Modifying other adjustable screen feature Display brightness 1. Press Monitor Setup and select Display brightness. Parameter colors Parameter color options include: yellow, white, green, red, blue, orange and violet. To change the color perform the following steps: 1. Press the Monitor Setup key. 2. Select Install/Service and enter the password. 3. Select Colors. 4. Select the desired parameter and the color. 2. Select from 10 to 100 %.
PAGE 36 32 Using modes The monitor has seven user modes. These user modes are predefined combinations of settings. These user modes determine what is displayed on the screen, in trends and alarm settings. User modes can also be customized and saved. The monitor starts in start-up mode, which is one of the user modes chosen during configuration.
PAGE 37 Setting trends Changing default trend Configuring snapshots You can select graphical or numerical trends to be displayed by default: 1. Press Monitor Setup key. 2. Select Install/Service and enter the password. 3. Select Trends & Snapshot. 4. Select Default Trend for Graph or Num. You can change the snapshot settings, 1. Press Monitor Setup key. 2. Select Install/Service and enter the password. 3. Select Trends & Snapshot 4.
PAGE 38 34 Setting time and date Changing the monitor installation settings NOTE: If the monitor is connected to the central station, it follows the central station’s time settings and the Time and Date menu is not available. Changing unit NOTE: You cannot change the monitor’s time settings after the patient has been admitted. 1. Press Monitor Setup and select Time and Date 2. Turn and push the Trim Knob to set the time and date. Battery setup Through this menu, you can check the battery status: 1.
PAGE 39 Changing alarm options 1. Press Monitor Setup key. Changing the printer settings 1. Press Monitor Setup key. 2. Select Install/Service and enter the password. 3. Select Installation – Alarm Options. • Show Limits: Select YES to show alarm limits in digit fields. YES is the default. • Show Audio ON/OFF: Select YES to enable alarm audio off. Select NO (default) disables audio off option in the Audio ON/ OFF menu in Alarms Setup.
PAGE 40 36 Setting time zone Time Zone menu is enable only when monitor is not connected to network and discharge paitent/haven’t admitted patient. 1. Press Monitor Setup key. 2. Select Install/Service and enter the password. 3. Select Time Zone • Daylight Saving: to turn on, turn off or set auto adjust daylight savings time (DST). • DST Offset Hour: to set the hours offset for daylight savings. • DST Offset Minute: to set the minutes offset for daylight savings. • DST Begins: to set daylight savings begin time.
PAGE 41 Alarms • Safety precautions • Warnings • • • • • • • • • • Verify alarm processing is active and no arrhythmia occurred during power interruption. Latched alarms are not retained through monitor reset if alarm condition is been removed. Do not rely on secondary alarm system for receipt of alarm signal. Observe patient frequently while alarms or audio are paused or off. No alarm sound during Audio Pause. The audible alarm signal can be paused by central station.
PAGE 42 38 Overview The monitor provides visible and audible indications of patient or system related alarms conditions. NOTE: If the monitor is connected to the network, it also sends alarms to the central station. 1. Alarm messages appear in the message field in the order of priority. 2. The alarming measurement value flashes (except for low priority alarms) and the color indicates the alarm category. 3. In some cases, a message in the digit or waveform field gives more detailed information. 4.
PAGE 43 Alarm categories The alarms are classified into four categories according to the priority depends primarily on the cause and alarm duration. The priority increasing with the duration and according to the physiological significance. NOTE: Asystole, ventricular fibrillation and V Tach alarms are always high priority alarms.
PAGE 44 40 Alarm conditions Alarm priority escalation • An escalating alarm starts at a designated priority level (low or medium) and will escalate to the next higher priority level of alarm (after a set number of seconds) if the alarm condition has not been resolved. It is important to note that these escalate up to the next level but will not reset until the condition has been resolved.
PAGE 45 Alarm activation Checking alarm function Physiological alarms have individual activation criteria as shown in the table. Alarm annunciation does not depend on case activity. Parameter Physiological alarm activation criteria ECG Active measurement for 30 seconds. Impedance respiration Active measurement for 30 seconds. Apnea After 3 breath within 1 minutes. SpO2 After successful pulse search. NIBP Manual, Auto or Stat mode started. IBP Active measurement for 30 seconds.
PAGE 46 42 Adjusting limits 1. Press the Alarms Setup key and select Adjust Limits. 2. 3. 4. 5. Turn the Trim knob to selest the specific alarm to be configured. Choose the highlight measurement. Push the Trim knob to open an adjustment window. Turn the Trim knob to change limits and accept them by pushing it. Move between selections by turning the Trim knob.
PAGE 47 Audible alarms off behavior When audible alarms are turned off: • All audible alarms are turned off except for specific alarms configured to break through the audio off setting. • The audio off bell icon displays in the upper left corner of the display screen. • The visual alarm signals still display. Turning audible alarms on/off 1. Press the Alarms Setup key and select Audio ON/OFF. If this option is not selectable, see “Changing alarm options.” 2. Select an alarm group.
PAGE 48 44 Pause audio and alarm reset behaviors Action Result Press audio pause key once • • Indicator Start a 2 minute audio pause period for all • alarms except the critical breakthrough alarms • (for example, V Tach). See the “Default configuration worksheet” for • details about breakthrough alarm Cease all latched alarms (including message and light).
PAGE 49 • • • • • • • • • • • • • • • • • • • NIBP cuff occlusion Check NIBP Weak pulsation Long measurement time NIBP manual NIBP cuff overpressure NIBP call service error Gas measurements removed Recorder module removed Entropy measurement removed Alarm volume changed Zero ICP separately Recorder system error Recorder cover open Recorder input voltage high Recorder input voltage low Recorder out of paper Recorder thermal array overheat No battery backup Showing alarm history 1. Press the Pt.Data & Trends key.
PAGE 50 46 Breakthrough Alarms The breakthrough alarms feature allows some alarms to “break through” (interrupt) an All Alarms Audio Off or a 2 minute alarm audio pause condition. See “Changing alarm options” in “Setting up the monitor before use” chapter for how to turn on/off breakthrough alarms feature. See the “Default configuration worksheet” delivered with the monitor for details of which alarms and conditions are enable for breakthrough feature.
PAGE 51 Starting and ending Safety precautions Starting monitoring Warnings 1. Prepare the patient connections according to the setup picture in the measurement section. Use only approved supplies and accessories, see the “Supplies and Accessories” catalog. The alarms and parameter settings become active. 2. Enter or load patient data by pressing the Admit/Discharge key, according to the instructions given later in this chapter. 3. Start the measurement according to the instructions in the measurement section.
PAGE 52 48 Entering patient data Loading patient data When you admit a patient, you must enter all relevant data: 1. Press the Admit/Discharge and select Admit Patient. 2. Choose the Patient Type (The option A/P means Adult/Pediatric, the option NEO means Neonatal). 3. Enter patient name, ID by selecting the letters or numbers, pushing to confirm and turning the Trim knob to the following character selection. 4. Select Demographics, enter Height, Weight and Age. The BSA is automatically calculated. 5.
PAGE 53 Saving data Ending monitoring The monitor continuously saves patient data, such as trends. Saving is activated once the patient is admitted. The monitor saves automatically: − In the monitor memory the most recent patient data up to 72 hours. − In the network the most recent patient data up to 2 to 90 days in central station depending on the configuration. 1. Print necessary data: press the Print/Record key. 2. Wait until the printing is finished.
PAGE 54 50 Demo Mode Troubleshooting The Demo Mode is designed for training and demo of operation before use. Under Demo Mode, the monitor displays the main vital signs values and waveforms. No need accessories, central station or any other peripheral equipment connect to the monitor while in Demo Mode. • The measured values are not displayed: − Check that you have selected the desired parameter to a waveform or digit field, see “Modifying the screen setup.” • You cannot perform a measurement or a function.
PAGE 55 Trends and Snapshot Cautions Trends view • (1) (2) (3) (4) (5) Snapshot waveforms are in some cases drawn from compressed data that may not allow perfect reconstruction. Verify diagnostic waveform measurements with the waveform data from realtime graph strips.
PAGE 56 52 Viewing and printing graphical trends To view graphical trends 1. Press the Pt.Data & Trends key. 2. Select Trends – Graphical. • To see more parameters, select Scroll Pages and scroll with the Trim Knob. • To see more data, select Cursor and scroll the page left and right with the Trim Knob. Graphical trends contain four trend pages each having up to six preconfigured fields with different parameters. Five fields can be displayed, and six fields can be printed.
PAGE 57 Take snapshots Viewing and printing snapshots A snapshot is a frozen frame of preconfigured waveforms or trends saved in the monitor memory. You can take up to 10 snapshots. It is automatically numbered. When graphical trend view, snapshots mark “s” in mark field; when numerical trends view, the number appears in the column ‘Mark’. To view snapshots: 1. Press the Pt.Data & Trends key. 2. Select Trends – Snapshot – Next Snapshot. Turn the Trim knob to move to the next snapshot.
PAGE 58 54 OCRG View realtime OCRG The monitor supports 8 minutes OCRG (oxycardiorespirogram) function in the NEONATAL mode. The OCRG subsystem provides services to view and review specific high resolution trends, high resolution beat-to-beat HR, high resolution beat-to-beat SpO2 and compressed respiration waveform – simultaneously in the same view. Two types of views are provided: OCRG Snapshot view and OCRG Realtime view. To display the realtime OCRG. 1. In NEONATAL mode, Press the Pt.Data & Trends key. 2.
PAGE 59 Printing and recording You need − Laser printer for printouts (PCL5 compatible, min. 2Mb memory) NOTE: Network printer only. − Optional recorder configuration for recording − Thermal paper for the recorder Printing with a laser printer You can print to the network connected laser printer from central station. If the monitor connect to S/5 network, you also can print with a laser printer from monitor directly, see following instruction. Selecting a printer 1. Press the Print/Record key.
PAGE 60 56 Printing currently displayed screen contents You can print currently displayed trend data. To print trend data: • Press the Pt.Data & Trends key and select: − Trends – Print Page Recording with the recorder Recording waveforms You can record three waveforms to a local recorder, and two to four waveforms to a network recorder: 1. Press the Print/Record key, select Record Waveforms. 2. Setup the record settings: • Waveform X: which waveform to be recorded.
PAGE 61 Recording numerical trends Recording graphical trends 1. Press the Print/Record key, select Record Trends You can record the current trends values of measured parameters. 1. Press the Print/Record key, select Record Trends. 2. Select Num Trend Type and Num. or Tab. to set up the format for the recorded numerical trends 3. To start the recording, select Record Numerical. 4. To stop recording, select Stop Numerical. 2. Select Graphic. Trend 1 or Graphic. Trend 2 to set up parameters of graphical trends. 3.
PAGE 62 58 Troubleshooting • • Printing is not possible: − Check the printer setting through Print/Record – Printer Connection. − Check that the printer is connected to the network. − Check the network cable. Recording is not possible: − Check the Central recorder if you are recording through network.
PAGE 63 Cleaning and care − Allow sensor and cable to dry completely after cleaning. Safety precautions Warnings • • • • • • • • • • Disconnect equipment from power line before cleaning If liquid has accidentally entered the system or its parts, disconnect the power cord from the power supply and have the equipment serviced by authorized service personnel. Regular preventive maintenance should be carried out annually.
PAGE 64 60 Cautions • • • PACKAGING DISPOSAL – Dispose of packaging material, observing applicable waste control regulations. Do not use or store equipment outside the specified temperature, humidity, or altitude ranges. DISPOSAL – At the end of its service life, the product described in this manual, as well as its accessories, must be disposed of in compliance with the guidelines regulating the disposal of each product.
PAGE 65 Permitted cleaning agents General cleaning instructions The exterior surface can be cleaned with the following disinfecting and sterilizing agents: To clean the monitor, module, displays, and other parts, complete the following procedure: 1. Turn off the monitor. 2. Disconnect the power cord. 3. Remove all cables and batteries (if applicable) and close battery door(s). 4. Use a soft, lint-free cloth with one of permitted cleaning agents to wipe the exterior surface. 5.
PAGE 66 62 Cable and leadwire cleaning instructions Gas module water trap cleaning instructions To clean ECG trunk cables, NIBP cuff and cables, and other reusable sensors, complete the following procedure: 1. Remove cables and leadwires from the device before cleaning. 2. Do not pull the long wires from the connector ends. Metal connections can be pulled away from the connectors. 3. For general cleaning of cables and leadwires, wipe with a cloth moistened with a mild soap and water solution. 4.
PAGE 67 Conditioning the batteries Changing fuses Condition the batteries regularly to maintain their useful life. Condition a battery every six months or when the message ‘Condition Battery A’ or ‘Condition Battery B’ displays. The monitor displays battery status messages and symbols. You can also check the battery status through Monitor Setup – Battery Setup. For more information, see “Replacing the batteries”, “Symbols” and “Messages.” 1. Remove the power cord if used. 2. Remove the fuse holder. 3.
PAGE 68 64 Regular checks When you start monitoring, check that: Entropy • • • • • The module is firmly in place. Accessories are intact and properly connected. The appropriate parameters display in the digit and waveform fields. ECG and impedance respiration • After connecting the ECG cable, check that the message ‘Leads off’ displays in waveform field . NIBP • After connecting NIBP cable, check that the message ‘Adult/ Pediatric’ or ‘Neonatal’ displays in NIBP digital field for several seconds.
PAGE 69 Calibrating Calibrating airway gases Before calibration 1. Ensure that the module is connected to the monitor. Ensure that you have an appropriate water trap in use. 2. Turn on the monitor. Let the monitor warm up for 30 minutes. 3. Attach a regulator to the calibration gas container. 4. Connect a new gas sampling line to the sampling line connector in the water trap. 5. Connect the other end of the gas sampling line to the regulator on the gas container. Leave the regulator overflow port open to room air.
PAGE 70 66 NOTE: The message ‘Zero error’ displays in case the zeroing fails. NOTE: The message ‘Calibration failed’ displays, if you do not start feeding gas within one minute after the automatic zeroing is complete, or if the calibration fails due to a large gain adjustment. NOTE: If zeroing or calibration failed, select Recalibrate to restart the calibration procedure.
PAGE 71 ECG • Safety precautions Warnings • • • • • • • Make sure that the leadwire set clips or snaps do not touch any electrically conductive material including earth. Whenever patient defibrillation is a possibility, use non-polarizing (silver/silver chloride construction) electrodes for ECG monitoring. Single-use devices and accessories are not designed to be reused. Reuse may cause a risk of contamination and affect the measurement accuracy.
PAGE 72 68 NOTE: Keep the ECG cable, lead set, and module connectors dry. Avoid excessive use of liquids when cleaning cables and connectors. ECG equipment to patient connection (1) ECG connector (2) Multi-Link 5-lead ECG trunk cable, or 3-lead ECG cable (3) 3 or 5 leadwire set NOTE: In 5-lead ECG, place the 5th electrode (C/V) in one of the six places indicated, and select the corresponding V lead label.
PAGE 73 Connecting ECG leadwire sets to ECG trunk cables Applying the electrodes to the patient • 1. Place the electrodes on the prepared sites. 2. Stabilize the electrode and leadwire with a leadwire stress loop near the electrode. 3. Tape the stress loop to the patient (excluding neonates). • For 3-lead ECG, use the Multi-Link 3-lead ECG cable with integrated leadwires or connect a 3 leadwire set to the Multi-Link 3- or 5-lead ECG trunk cable.
PAGE 74 70 Selecting the ECG leads 1. Press the ECG key. Selecting the number of electrodes for 5-lead ECG 1. Press the ECG key. 2. Select a lead for ECG1 Lead, ECG2 Lead, or ECG3 Lead. 2. Select ECG Setup. 3. Select 5-lead Cable – 3elect or 5elect. With 3-lead ECG, only one lead (ECG1 Lead) can be selected. With 5lead ECG, you can select three leads.
PAGE 75 Selecting the ECG filter 1. Press the ECG key. Displaying the ECG grid 1. Press the ECG key 2. Select ECG Setup – Filter, and select the appropriate option: • STfilt: Filters high-frequency artifacts but catches slow ST changes. • Monit: Filters high-frequency artifacts and slow ST changes. • Diagn: Catches high-frequency changes and slow ST changes. 2.
PAGE 76 72 For your notes:
PAGE 77 Pacemaker detection Warnings • • • • • • Monitoring pacemaker patients 1. Press the ECG key. Do not diagnostically interpret pacemaker spike size and shape. A pacemaker pulse can be counted as a QRS during asystole in either pace mode. Keep pacemaker patients under close observation. The shape of QRS complex may be changed so much because of the pacemaker that QRS detection may be affected. The monitoring of pacemaker patients can only occur with the pace program activated.
PAGE 78 74 For your notes:
PAGE 79 Arrhythmia detection • NOTE: The VSP-C software only supports severe analysis, which detects asystole, bradycardia, tachycardia, ventricular fibrillation, and ventricular tachycardia. Warnings • LOSS OR DETERIORATION OF ARRHYTHMIA DETECTION Automated arrhythmia analysis programs may incorrectly identify the presence or absence of an arrhythmia. A physician must therefore interpret the arrhythmia information in conjunction with other clinical findings.
PAGE 80 76 to find the best match between each incoming complex and the set of stored (learned) templates. If no match is found with the existing template, a new template is stored for the identified new QRS shape. Incremental template updating allows information from each beat, that correlates over time, to be reflected in the associated template.
PAGE 81 ST Detection Manually adjusting ST measurement points The monitor analyzes ST for all measured leads and displays ST trends separately for each lead. ST analysis starts automatically after the leads have been connected and the QRS detection has started. The J, ISO and ST measurement points can also be selected manually. If any the measurement points are manually selected, the other two measurement points are set to the current values. 1. Press the ECG key 2. Select Adjust ST. 3.
PAGE 82 78 About the ST segment measurement algorithm The ST segment begins at the point where the QRS ends (J point). Diagnostic criteria of ST segment changes are measured at 60 ms after the J point. For monitoring purposes it is important to keep the measurement point fixed during monitoring to notice the ST changes on the respective trends. The sophisticated algorithms of monitor search the J and isoelectric (ISO) points. The system learns the ECG and stores the reference QRST complex.
PAGE 83 Impedance respiration Safety precautions Warnings • • • • • • • • Make sure that the leadwire set clips or snaps do not touch any electrically conductive material including earth. The monitor may not detect all episodes of inadequate breathing, nor does it distinguish between central, obstructive and mixed apnea events. ELECTRODE CONFIGURATION – Impedance respiration monitoring is not reliable when ECG electrodes are placed on the limbs.
PAGE 84 80 Respiration equipment to patient connection Use the same setup as in the ECG measurement, see “ECG” section. NOTE: Impedance respiration measurement with neonates is not available in specific regions, including USA, Argentina, Guam, Puerto Rico, Saint Croix, Saint Thomas, and Canada. Turning on the respiration measurement Select respiration to a waveform or digit field, otherwise respiration data is not included in trends and no alarms are activated. 1. Press the Others key. 2.
PAGE 85 Adjusting the no breath time If the monitor has the NeoResp license, in the neonatal mode, the impedance respiration can set up the detect time for alarm “No Breath”. 1. Press the Others key 2. Select Resp Setup – No Breath Time The default detection time is 15s. Adjusting respiration alarms 1. Press the Others key. 2. Select Resp Setup – Resp Rate Alarm. • To set up the limits, select Adjust Limits. • To OFF/ON the limits, select RR Alarm. 3.
PAGE 86 82 For your notes:
PAGE 87 Pulse oximetry (SpO2) Safety precautions Warnings • • • • • • Allow sensor and cable to dry completely after cleaning. Moisture and dirt on the connector can affect the measurement accuracy. To prevent erroneous readings, do not use physically damaged sensors, cables or modules. Discard a damaged sensor or cable immediately. Never repair a damaged sensor or cable; never use a sensor or cable repaired by others. A damaged sensor or a sensor soaked in liquid may cause burns during electrosurgery.
PAGE 88 84 • • • • • • • A pulse oximeter should be considered an early warning device. As a trend toward patient deoxygenation is indicated, blood samples should be analyzed by a laboratory CO-oximeter to completely understand the patient’s condition. Check that the pulse oximetry waveform is physiological in shape to ensure waveform quality and minimize noise spikes caused by motion conditions. (Not applicable when monitoring SpO2 with Masimo SET technology). Interfering substances can affect the SpO2 reading.
PAGE 89 SpO2 to patient connection (1) (2) (3) (4) Compatible SpO2 measurement capability Interconnect cable Reusable sensors Disposable sensors NOTE: For a comprehensive list of accessories, see the “Supplies and Accessories” catalog delivered with the monitor. NOTE: For each SpO2 accessory, refer to the instructions for use in the accessory package for patient weight limits.
PAGE 90 86 Preparing the patient for a SpO2 measurement Adjusting SpO2 pulse beep tone volume 1. Connect the sensor to the blue connector in the monitor. 2. Clean the surface of reusable sensors. 3. Clean the application site. Remove nail polish, artificial fingernails, earrings etc. 4. Clip long fingernails. 5. Attach the sensor cable to the wrist or bedclothes to prevent the cable and sensor from moving. 6. Check that the red light is in the sensor. 7.
PAGE 91 Adjusting the Masimo SpO2 averaging time Stopping the SpO2 measurement NOTE: For SpO2 modules with Masimo technology and Masimo sensors. 1. Press the SpO2 key. 2. Select Averaging. 1. Remove the SpO2 sensor from the patient. 2. Disconnect the sensor cable from the monitor. 3. Select Audio Pause key to close the ‘SpO2 probe off’ alarm. Setting SpO2 alarms 1. Press the SpO2 key. Adjusting the Masimo SpO2 sensor sensitivity level NOTE: For SpO2 modules with Masimo technology and Masimo sensors. 1.
PAGE 92 88 GE Trusignal technology clinical studies on neonatal GE Oxy-AF and GE Oxy-SE sensors have been validated for neonatal accuracy. The subject demographics included 28 neonates and 1 infant (15 females and 14 males). The subjects ranged in age from newborn to 37 days old. The weights ranged from 560 to 3060 g. The skin tones included in the study were light to dark. For neonatal study, the Arms of the collected convenience samples are 2.
PAGE 93 Non-invasive blood pressure (NIBP) • Safety precautions Warnings • • • • • • • • • The NIBP parameter will not measure blood pressure effectively on patients who are experiencing seizures or tremors. Arrhythmias will increase the time required by the NIBP parameter to determine a blood pressure and may extend the time beyond the capabilities of the parameter. Do not apply external pressure against the cuff while monitoring. Doing so may cause inaccurate blood pressure values.
PAGE 94 90 NIBP to patient connection (1) (2) (3) (4) NIBP connector in monitor Cuff hose Cuff of correct size Brachial artery arrow (printed on cuff) (5) Cuff index line (printed on cuff) NOTE: For a comprehensive list of accessories, see the “Supplies and Accessories” catalog delivered with the monitor.
PAGE 95 Preparing the patient for a NIBP measurement To produce a single NIBP measurement 1. Select an appropriate NIBP cuff size for the patient. 2. Connect the NIBP cuff hose to the module’s NIBP connector. 3. To position the NIBP cuff on the patient: • Place the cuff arrow over the brachial artery (or whatever artery is being used). • Make sure that the cuff index line falls within the range markings on the cuff. • Wrap the cuff around the limb. 4.
PAGE 96 92 To produce automatic NIBP measurements To automatically measure NIBP at set time intervals, you must first set the cycle time before setting the automatic measurements. Starting and stopping NIBP Auto • You also can configure a custom auto mode to meet the need of your clinical situation. Setting cycling intervals 1. Press the NIBP key. 2. Select Cycle Time. 3. Select the interval time from the list with the Trim Knob.
PAGE 97 Adjusting the NIBP measurement completion tone volume 1. Press the NIBP key. During measurement • 2. Select NIBP Setup. 3. Set the Ready Prompt • Setting NIBP alarms 1. Press the NIBP key. • 2. Select NIBP Alarm. 3. Select Adjust Limits to set up the limits. 4. Select Sys Alarm, Dia Alarm or Mean Alarm to OFF/ON the limits. 5. You can also select Alarms Setup to adjust NIBP or related alarms. • Observe the cuffed limb frequently. Measurement may impair blood circulation.
PAGE 98 94 Principles of SuperSTAT Noninvasive Blood Pressure Determination The oscillometric method of determining NIBP is accomplished by a sensitive transducer which measures cuff pressure and pressure oscillations within the cuff. For the first determination taken on a patient, the algorithm stores the pattern of the patient’s oscillation size as a function of the pressure steps.
PAGE 99 Invasive blood pressure Safety precautions Warnings • • • • • All invasive procedures involve risks to the patient. Use aseptic technique. Follow catheter manufacturer’s instructions. Make sure that no part of the patient connections touches any electrically conductive material including earth. Mechanical shock to the invasive blood pressure transducer may cause severe shifts in zero balance and calibration, and cause erroneous readings.
PAGE 100 96 IBP equipment to patient connection (1) (2) (3) (4) (5) (6) (7) Adapter cable for dual IBP measurement Compatible IBP measurement capability Fluid bag or bottle with pressure infusor Flushing set Disposable catheter Transducer Adapter cable for the IBP transducer You can monitor up to two pressure channels by using a dual cable. NOTE: For a comprehensive list of sensors and accessories, see the “Supplies and Accessories” catalog.
PAGE 101 Preparing the patient for a IBP measurement Set up ventilation mode 1. For the setup, prepare the transducer kit according to the manufacturer’s instructions. 2. Remove entrapped air from with the transducer setup. 3. Zero the transducer by opening it to air, pressing the IBP Zero All command board key or the IBP monitor key and selecting Zero Pressures – Zero ALL. You can zero one channel at a time with selection Zero IBP1 or Zero IBP2. Zero each channel. NOTE: Selecting Zero ALL does not zero ICP.
PAGE 102 98 Factory default descriptions The channels have the following factory default descriptions: LABEL IBP1, Art, ABP IBP2, CVP RAP, LAP ICP PA RVP UAC UVC Scale mmHg/kPa 200/30 20/4 20/4 20/4 60/8 60/8 100/14 10/2 Color Red Blue White White Yellow White Red White Alarm source Sys Mean Off Off Off Off Sys, Dia, Mean Mean Digit format S/D Mean Mean CPP S/D S/D S/D Mean Filter (Hz) 22 9 9 9 9 9 14 14 Response Normal Normal Normal Normal Normal Normal N
PAGE 103 Setting IBP alarms 1. Press the IBP key. 2. Select IBP1 Setup – IBP1 Alarm and IBP2 Setup – IBP2 Alarm. 3. Select Adjust Limits to set up the limits. 4. Select Sys Alarm, Dia Alarm or Mean Alarm to OFF/ON the limits. 5. You can also select Alarms Setup to adjust IBP or related alarms. Troubleshooting • • Readings seem unstable: − Make sure that there are no air bubbles in the transducer system. − Flush and zero. − Place the transducer on the patient’s mid-heart level and zero.
PAGE 104 100
PAGE 105 Temperature Safety precautions NOTE: Temperature measurement response time is affected by use of esophogeal stethoscope with certain temperature sensors. Warnings Temperature equipment to patient connection • Temperature measurement response time is affected by use of esophogeal stethoscope with certain temperature sensors.
PAGE 106 102 Preparing the patient for a temperature measurement Combining different temperatures 1. Follow the manufacturer’s instructions for probe application and instructions. 2. Connect the adapter cable to the acquisition module connector. The monitor can display the delta value between different temperatures if they are displayed in the same digit field. For example, to display T2 – T1: 1. Press the Monitor Setup key. 2. Select Screen Setup. 3. Select Digit Fields. 4.
PAGE 107 Airway gas • Safety precautions Warnings • • • • • • • • • • • • • • Always check the airway adapter for a tight connection and proper operation before attaching it to the patient. Remove the airway gas sampling line from the patient’s airway while nebulized medications are being delivered. Keep the monitor horizontal when the gas module is used. Tilting the monitor may cause erroneous results in the gas module’s readings and damage the module.
PAGE 108 104 • • • • • • • A failure in zeroing or calibrating airway gases may cause inaccurate readings. E-miniC module: O2, N2O and anesthetic agent gases may interfere with EtCO2 readings. EQUIPMENT FAILURE OR INACCURATE READINGS — Planned maintenance should be carried out annually according to the instructions given in the technical manual. Failure to implement the recommended maintenance schedule may cause equipment failure or inaccurate readings.
PAGE 109 Alternative airway gases modules The E-miniC, CARESCAPE Respiratory Module (E-sCO and E-sCAiO modules) and Airway Gas Option (N-CAiO module) provide airway measurements. Letters in these module name stand for: C=CO2 and N2O, O=patient O2, A=anesthetic agents and i=agent identification The following tables show the airway gases for each acquisition module. The “X” indicates that the module measures the listed parameter.
PAGE 110 106 E-miniC module to patient connection (1) (2) (3) (4) E-miniC module Gas sampling line Airway adapter with sampling line connector Sampling line connector on the water trap NOTE: For a comprehensive list of accessories, see the “Supplies and Accessories” catalog.
PAGE 111 CARESCAPE Respiratory Module or Airway Gas Option to patient connection (1) (2) (3) (4) E-sCO, E-sCAiO or N-CAiO module Gas sample, gas sampling line connector on the water trap Gas sampling line Gas sampling line connector on the airway adapter; place the connector upwards (5) Airway adapter with sampling line connector (6) Heat and moisture exchanger with filter (HMEF) (optional) 107
PAGE 112 108 Preparing for a gas measurement CO2 measurement 1. Make sure that the water trap container is empty and properly attached. 2. Connect the gas sampling line to the sampling line connector on the water trap. 3. Connect the sample gas outlet to gas scavenging if N2O or volatile agents are used. 4. Turn on the monitor. The monitor performs a self-check. Make sure the related gas measurement is ON. 5. Wait until the message ‘Calibrating gas sensor’ disappears. 6.
PAGE 113 Changing the units Setting alarms You can use %, kPa or mmHg as the CO2 measurement units. The units can be changed in the CO2 Setup menu: 1. Press the Airway Gas key. 2. Select CO2 Setup – Unit. 3. Choose the option. Setting CO2 alarms 1. Press the Airway Gas key. 2. Select CO2 Setup – CO2 Alarm. 3. Select Adjust Limits to set up the limits. 4. Select EtCO2 Alarm, FiCO2 Alarm to OFF/ON the limits. 5. You can also select Alarms Setup to adjust CO2 or related alarms.
PAGE 114 110 O2 measurement Anesthetic agent and N2O measurement NOTE: B40 only. NOTE: B40 only. NOTE: The E-sCAiO and N-CAiO modules automatically identify the agent being used. If the agent value is out of measurment range, “—” will be displayed on screen, which means the value is invalid. Selecting the O2 scale If the difference between FiO2 and EtO2 is above 6%, change the O2 scale: 1. Press the Airway Gas key. 2. Select O2 Setup. 3. Select Scale 4. Select an option from the scale list.
PAGE 115 Setting agent alarms 1. Press the Airway Gas key. 2. 3. 4. 5. Airway gases calculations • Select Agent/N2O Setup – Agent Alarm. Select Adjust Limits to set up the limits. Select EtAA Alarm, FiAA Alarm to OFF/ON the limits. You can also select Alarms Setup to adjust agent or related alarms. MAC= where AA1=primary agent, AA2=secondary agent, x(AA) is Hal=0.75%, Enf=1.7%, Iso=1.15%, Sev=2.05%, Des=6.0% and N2O=100%. • Minimum Alveolar Concentration (MAC) and balance gas NOTE: B40 only.
PAGE 116 112 To select the MAC type 1. Press Monitor Setup key. 2. Select Install/Service and enter the password. 3. Select Installation – Monitor Settings – Parameter Settings. 4. Select MAC Type. To enable the MACage calculation: − Give the patient’s age in menu Admit/Discharge – Admit Patient – Demographics. − Attach a temperature sensor. If the patient’s age is not given, the monitor shows normal MAC even if MACage has been selected.
PAGE 117 During monitoring Points to note • • • Empty the water trap container when half full. Follow local hospital’s regulations to dispose the accumulated fluids. Disconnect the airway adapter during nebulization of medications. NOTE: When the measured CO2 value is outside the specified measurement range, the numeric value is gray.
PAGE 118 114 Disposal of gases Troubleshooting When N2O and volatile anesthetics are used, prevent operating room pollution by connecting the sample gas outlet (gas exhaust) of the module to the scavenging system. Follow local hospital’s regulations. • Scavenging through the ventilator reservoir 1. Connect an exhaust line to the sample gas outlet (gas exhaust) on the module’s front panel. 2. Attach the other end of the line to the ventilator reservoir.
PAGE 119 Entropy Note Safety precautions • Warnings • • • • Make sure that the electrodes, sensor and connectors do not touch any electrically conductive material, including earth. DEFIBRILLATOR PRECAUTIONS – Patient signal inputs labeled with the CF and BF symbols with paddles are protected against damage resulting from defibrillation voltages. To ensure proper defibrillator protection, use only the recommended cables and leadwires. Do not reuse the single-use entropy electrodes.
PAGE 120 116 Entropy equipment to patient connection Entropy module keys (1) (2) (3) (4) There are two keys on the module: Module with Entropy measurement capability GE Entropy sensor cable GE Entropy sensor, or Entropy sensor NOTE: A portion of the Entropy software is derived from the RSA Data Security, Inc. MD5 Message-Digest Algorithm. NOTE: Entropy sensors are latex and PVC free, disposable and for single-patient use only.
PAGE 121 Preparing for Entropy measurement Selecting the EEG scale 1. Connect the Entropy sensor cable to the module. 2. Clean the application site according to the sensor’s instructions for use and let it dry before attaching the sensor. 3. Place the Entropy sensor on the patient’s forehead; see sensor package for instructions. 4. Connect the sensor to the Entropy sensor cable. 5. Observe the results of the automatic sensor check in the parameter window. 6.
PAGE 122 118 Using the manual Entropy sensor check Using the automatic Entropy sensor check Whenever required, you can perform the sensor check manually. 1. Press the Check Sensor module key, or: − Press the Entropy module key, or press the Others key, select Entropy − Select Check Sensor. 2. Observe the results on the screen. Do not press the sensor during the check to avoid signal noise. 3. The measurement continues automatically after the sensor has passed the check.
PAGE 123 Setting Entropy alarms 1. Press the Entropy module key, or press the Others key, select 2. 3. 4. 5. Troubleshooting • Entropy Select Entropy Alarms. Select Adjust Limits to set up the limits. Select RE Alarm, SE Alarm to OFF/ON the limits. You can also select Alarms Setup to adjust Entropy or related alarms. • Stopping the Entropy measurement • 1. Remove the Entropy sensor from the patient. 2. Disconnect the sensor from the sensor cable. 3. Discard the sensor.
PAGE 124 120 For your notes:
PAGE 125 Troubleshooting NOTE: Always check the patient’s condition first in problematic situations or if an alarm is triggered. See also “Messages.” Also note that if the measurement or function does not appear on the screen, check module connections. Airway gases Batteries Values are too low: • Check the sampling line and connectors for leakage. • Check the patient status. Values are too high: • Check the sampling line for blockage. • Check the patient status.
PAGE 126 122 Entropy Invasive pressures Entropy values seem unstable: • Check that the sensor is not dried out. • Check the sensor attachment and placement. • Check the patient status. Entropy EEG signal is noisy: • Remove disturbing equipment from the proximity of the Entropy module or sensor. • Check the sensor’s contact with skin. • Check electrodes. Entropy EEG signal is poor: • Check the sensor’s contact with skin. • Check electrodes.
PAGE 127 Printing Pulse oximetry Printing is not possible: • Check the printer setting through Print/Record – Printer Connection. • Check that the printer is connected to the network. • Check the network cable. SpO2 signal is poor: • Check the sensor and sensor positioning. • If in GE SpO2 configuration, change the SpO2 Response (averaging time) to Normal. • Note that skin pigment causes differences. • Make sure that the patient is not moving.
PAGE 128 124 Messages Always check the patient first. If any problem or message persists, contact qualified service personnel. Messages are listed here in alphabetical order. • Asystole • Agent Mixture − Check the patient status. − Mixture of halogenated agents is detected. Check the − Check the electrodes. ventilator and agent vaporizer settings. • Battery A temperature high, Battery B temperature high • Alarm setup changed from Central − Replace the battery.
PAGE 129 • Check SpO2 probe − SpO2: Check the sensor and connections. • Check sample gas out − Gases: Remove blockage from the sample gas outlet. • Condition Battery A, Condition Battery B − Condition the battery according to the instructions of the external charger. • Creating OCRG snapshot − In neonatal mode, the value of HR, SpO2 or Apnea time is out of limits. • Default settings returned. − Check your power and reset the settings.
PAGE 130 126 • − Network recorder has been started. Please wait until the Long measurement time − NIBP: reduce patient’s motion. recording is finished. • NIBP call service error − NIBP: Contact authorized service personnel. • NIBP manual − Check the cuff and cuff hose. • NIBP cuff overpressure − Check NIBP cuff hose and tubes. − Restart measurement. Mode Data Reset − Contact authorized service personnel. • NIBP cuff loose − Check the cuff and cuff hose.
PAGE 131 • No printer selected − Select a printer, see the “Printing and recording“ chapter. • Recorder: cover open − Close the recorder cover. • Patient discharged − Discharge patient. • Recorder: input voltage high, Recorder: input voltage low − Contact authorized service personnel. • Patient admitted − Admit patient. • Recorder: thermal array overheat − Contact authorized service personnel. • P1 over range/P2 over range − Check the patient status.
PAGE 132 128 • Select inflation limits − NIBP: You are using a hose without an automatic identification. Select appropriate inflation limits. NOTE: AUTO option is not available for these hoses. • Snapshot created − A snapshot is created manually or on alarms. • Snapshot memory full. Oldest snapshot erased. − The oldest snapshot lost. • • SpO2 probe off − Check connection between sensor and patient. − Replace the sensor. SpO2 faulty probe − Replace the sensor.
PAGE 133 Abbreviations /min °C °F µg beats per minute, breaths per minute Celsius degree Fahrenheit degree microgram A A a a/AO2 AA AaDO2 AAMI alveolar arm (describing location) arterial arterio-alveolar PO2 ratio anesthetic agent alveolo-arterial oxygen difference Association for the Advancement of Medical Instrumentation arterial blood gases arterial pressure Anesthesia Delivery Unit auditory evoked potential airway temperature alpha frequency band Anesthesia Monitor amplitude anterior apnea arrhythmia arterial
PAGE 134 130 CMRR CO CO2 COHb Compl Cont. Contrl Core Count CPB CPP CSA CT CvO2 CVP common mode rejection ratio carbon monoxide carbon dioxide carboxyhemoglobin compliance continuous controlled ventilation core temperature count of responses cardiopulmonary bypass cerebral perfusion pressure compressed spectral array computer tomography (mixed) venous oxygen content central venous pressure d dB DBS DEL Delta, De depr. Des Dia Diagn DIFF DIS DO2 DO2I DSC Dyn.
PAGE 135 FiO2 Flow Freq. ft FVloop fraction of inspired oxygen airway gas flow frequent foot, feet flow volume loop G g Graph.
PAGE 136 132 MetHb MF mg Min min ml MLAEP mmHg mol Monit MRI Mult. Multif.
PAGE 137 Pleth PM non-capt. PM non-funct.
PAGE 138 134 SVR SVRI SVV Sys systemic vascular resistance systemic vascular resistance index stroke volume variation systolic pressure T T corr. T inj. T t T(BTPS) T1% T1, T2 Tab. Tachy Tbl, Tblood Temp Theta, Th TOF TOF% Trigem.
PAGE 139 Technical specifications WARNING: Operation of the monitor outside the specified values may cause inaccurate results. NOTE: Information in this section may be especially useful to clinicians.
PAGE 140 136 Operating altitude 660 to 1060 mbar Power comsumption Standby: ≤1.2 W Printing: ≤10 W Recorder type Thermal array Resolution Vertical 8 dots/mm (200 dots/inch) in non-waveform mode Horizontal 24 dots/mm (600 dots/inch) mimimum in waveform mode Paper width 50 mm, printing width 48 mm Waveforms Selectable 1, 2, or 3 waveforms Print speed 1, 6.25, 12.
PAGE 141 ECG specifications Leads available 3-lead configuration: I, II, III 5-lead configuration: I, II, III, aVR, aVL, aVF and VA QRS detection range 0.5 to 5mV QRS detection width (Q to S) 40 to 120 ms Defibrillation protection 5000 V, 360 J Recovery time <5 s Input impedance Common mode > 10 M Ω@ 50/60 Hz Differential > 2.5 M Ω from 0.67 to 40 Hz Common mode rejection 90 dB minimum at 50 Hz Tall T wave rejection >1.
PAGE 142 138 Figure 4b halved amplitude: 7.0 s (6.1 to 7.5 s) Figure 4b normal amplitude: 5.8 s (4.5 to 7.4 s) Figure 4b doubled amplitude: 6.1 s (5.1 to 7.0 s) Direct cardiac application: The display area reserved for the ECG measurement in the monitoring system screen may not be adequate for displaying the complete ECG amplitude when measuring ECG direct from the surface of the heart. Clipping of the signal can be reduced by adjusting the size of the signal on the screen (for example, from the default 1.
PAGE 143 Measurement accuracy GE TruSignal SpO2 specifications Measurement and display range 1 to 100% Display resolution 1 digit (1% of SpO2) Display averaging Normal 12s, Fast 3s Calibrated against functional oxygen saturation.
PAGE 144 140 Table 1: Accuracy for sensors (Arms) * GE SpO2 Sensor Non motion (70-100%) Motion (70-100%) Low perfusion (70-100%)** Neonatal (70-100%) TS-E-D, TS-E2-GE, TS-E4-GE ±3 digits unspecified unspecified unspecified TS-SE-3 ±2 digits unspecified unspecified ±3 digits TS-F-D, TS-F2-GE, TS-F4-GE, TS-SA-D, TS-SA4-GE ±2 digits unspecified ±3 digits unspecified TS-W-D ±2 digits unspecified unspecified unspecified TS-AP-10, TS-AP-25 ±2 digits ±3 digits unspecified unspecified TS-AF-10,
PAGE 145 Table 2: The table below shows Arms values measured using GE SpO2 sensors with GE CARESCAPETM V100 in a clinical study.* GE SpO2 Sensor 70 – 80% 80 – 90% 90 – 100% OXY-E 2.3 digits 1.4 digits 1.3 digits OXY-SE 2.5 digits 2.0 digits 1.1 digits OXY-F 1.3 digits 1.0 digits 1.1 digits OXY-W 2.9 digits 1.8 digits 1.0 digits OXY-AP 2.0 digits 1.9 digits 1.7 digits OXY-AF 2.5 digits 1.4 digits 0.
PAGE 146 142 Nellcor SpO2 specifications Masimo SpO2 specifications Measurement range 1 to 100% Measurement range 1 to 100% Display range 0 to 100% Display range 0 to 100% Calibrated against functional oxygen saturation. Calibrated against functional oxygen saturation.
PAGE 147 * This information may be useful to clinicians, such as those performing NIBP photodynamic therapy. Invasive blood pressure Measurement range -40 to 320 mmHg (-5.3 to 42.
PAGE 148 144 Temperature Airway gases Measurement units ° Fahrenheit (F) ° Celsius (C) Accuracy specifications apply in normal conditions after a 30 min warm-up period: Measurement range 10 to 45°C (50 to 113°F) Ambient temperature 18 to 28°C, within ±5°C of calibration Measurement accuracy ± 0.1°C without temperature sensor Ambient pressure Display resolution ± 0.
PAGE 149 Measurement accuracy E-miniC module • E-sCO, E-sCAiO, N-CAiO • module Resolution CO2 display resolution 4 to 20 breaths/min: ±1 breaths/ min 20 to 80 breaths/min: ±5% 4 to 20 breaths/min: ±1 breaths/ min 20 to 100 breaths/min: ±5% CO2 total system response time • E-miniC module • E-sCO, E-sCAiO, N-CAiO module 1 breaths/min CO2 CO2 measurement range • E-miniC module E-sCO, E-sCAiO, N-CAiO • module CO2 measurement accuracy E-miniC module CO2 rise time • E-miniC module E-sCO, E-sCAiO, N-CAiO • module
PAGE 150 146 N2O measurement range 0 to 100 vol% Anesthetic agents rise time Hal, Enf, Iso, Des: < 420 ms with a 3 m sampling line Hal: < 800 ms with a 3 m sampling line Hal, Enf, Iso, Des: < 700 ms with a 6 m sampling line Hal: < 1800 ms with a 6 m sampling line N2O measurement accuracy 0 to 85 vol%: ± (2 vol% + 2% of reading) 85 to 100 vol%: ± (2 vol% + 8 % of reading) N2O display resolution 1% N2O total system response < 3.4 seconds with a 2 or 3m sampling line time Hal drift < 0.1 vol% Enf drift < 0. PAGE 151 Non-disturbing gases of which effect on CO2 (5 vol%) readings < 0.2 vol%: − − − − − − Disturbing gas and its effect Ethanol C2H5OH (<0.3%) Acetone (<0.1% ) Methane CH4 (<0.2%) Nitrogen N2 (0-100%) water vapor (0-100%) Trichloromonofluoromethane (<1%) − Dichlorotetrafluoroethane (<1%) − Dichlorofluromethane (<1%) • • • • • • • • • • • E-sCO, E-sCAiO, N-CAiO module 147 Halotane (4%): increases CO2 (5 vol%) < 0.3 vol% Isoflurane (5%): increases CO2 (5 vol%) < 0. PAGE 152 148 Non-disturbing gases: − − − − − − − − Ethanol C2H5OH (< 0.036%) Acetone (< 0.2%) Methane CH4 (< 0.3%) Isopropanol (< 0.48%) Nitrogen N2 Carton Monoxide CO (< 100 ppm) Nitrous Oxide NO (< 200 ppm) Freon R134A (< 1%) (for CO2, O2 and N2O) − Water vapor Effects of a nondisturbing gas to the measured gas concentrations • • • • CO2 < 0.2 vol% N2O < 2 vol% O2 < 2 vol% Anesthetic agents < 0.15 vol% Gas cross effects • Helium (50 vol%): Decreases CO2 (5 vol%) readings < 0. PAGE 153 Electromagnetic Compatibility Changes or modifications to this system not expressly approved by GE can cause EMC issues with this or other equipment. This system is designed and tested to comply with applicable regulation regarding EMC and must be installed and put into service according to the EMC information stated in this section. PAGE 154 150 Guidance and manufacturer’s declaration – electromagnetic immunity Guidance and manufacturer’s declaration – electromagnetic immunity The monitor is intended for use in the electromagnetic environment specified below. The customer or the user of the monitor should assure that it is used in such an environment. PAGE 155 Voltage dips, short interruptions and voltage variations on power supply lines IEC 61000-4-11 Power frequency (50/60 Hz) magnetic field IEC 61000-4-8 <5% UT (>95% dip in UT) for 0.5 cycle <5% UT (>95% dip in UT) for 0.
PAGE 156 152 Guidance and manufacturer’s declaration – electromagnetic immunity Guidance and manufacturer’s declaration – electromagnetic immunity The monitor is intended for use in the electromagnetic environment specified below. The customer or the user of the monitor should assure that it is used in such an environment.
PAGE 157 where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey, a should be less than the compliance level in each frequency range. b Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1At 80 MHz and 800 MHz, the higher frequency range applies.
PAGE 158 154 Recommended separation distances The table below provides the recommended separation distances (in meters) between portable and mobile RF communications equipment and the monitor. The monitor is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled.
PROCARE* Monitor B40/B20 Technical Reference · PDF filePROCARE* Monitor B40/B20 Technical Reference Manual … 1.6 Inserting and removing the E-miniC module … 5 Set/Test 32 g o
Conformity according to the Council Directive 93/42/EEC concerning Medical Devices
ClassificationsIn accordance with IEC 60601-1 Class I and internally powered equipment – the type of protection against electric shock. Type BF or CF equipment. The degree of protection against electric shock is indicated by
a symbol on each parameter module. Equipment is not suitable for use in the presence of a flammable anesthetic mixture with
air or with oxygen or nitrous oxide. Continuous operation according to the mode of operation. Portable Monitor
In accordance with IEC 60529 IP21 – degree of protection against harmful ingress of water.
In accordance with EU Medical Device Directive IIb.
In accordance with CISPR 11: Group 1 Class A;
Group 1 contains all ISM (Industrial, scientific and medical) equipment in which there is intentionally generated and/or used conductively coupled radio-frequency energy which is necessary for the internal functioning of the equipment itself.
Class A equipment is equipment suitable for use in all establishments other than domestic and those directly connected to a low-voltage power supply network which supplies buildings used for domestic purposes.
TrademarksDash, ProCare, DINAMAP, EK-Pro, Trim Knob, Unity Network, Datex, Ohmeda, S/5, D-fend, D-fend+, Mini D-fend, OxyTip+, EarSat, FingerSat, FlexSat are trademarks of GE Healthcare. All other product and company names are property of their respective owners.
키워드에 대한 정보 ge b40 monitor service manual
다음은 Bing에서 ge b40 monitor service manual 주제에 대한 검색 결과입니다. 필요한 경우 더 읽을 수 있습니다.
이 기사는 인터넷의 다양한 출처에서 편집되었습니다. 이 기사가 유용했기를 바랍니다. 이 기사가 유용하다고 생각되면 공유하십시오. 매우 감사합니다!
사람들이 주제에 대해 자주 검색하는 키워드 GE | Monitor B40 and B40i
- 동영상
- 공유
- 카메라폰
- 동영상폰
- 무료
- 올리기
GE #| #Monitor #B40 #and #B40i
YouTube에서 ge b40 monitor service manual 주제의 다른 동영상 보기
주제에 대한 기사를 시청해 주셔서 감사합니다 GE | Monitor B40 and B40i | ge b40 monitor service manual, 이 기사가 유용하다고 생각되면 공유하십시오, 매우 감사합니다.

